The national framework for regulation of gene technology
Australia has a nationally consistent regulatory framework for regulation of gene technology. This regulatory framework is a collaboration between all Australian states and territory governments.
The department is part of this nationally consistent regulatory system that underpins and enhances Australia’s and the States’ use of safe regulatory approved genetically modified organisms (GMOs).
Regulation of gene technology
The use of gene technology in WA is regulated under the National Gene Technology Regulatory Scheme, with constitutional power to regulate gene technology shared between the Australian Government and state and territory governments.
National consistency of the Scheme: Governance and regulations
In 2001 the states and territories signed the intergovernmental Gene Technology Agreement 2001, recognising the need for a consistent national scheme for the regulation of gene technology. The Commonwealth laws (Gene Technology Act 2000 and Gene Technology Regulations 2001) govern the national gene technology regulatory scheme.
The aim of the national Scheme is to protect human health, safety and the environment through risk identification, risk assessment and risk management.
For the Scheme to operate, Australian states and territories must have legislation that allows the Commonwealth gene technology legislation to be in effect as state law.
Legislative consistency benefits government and non-government stakeholders.
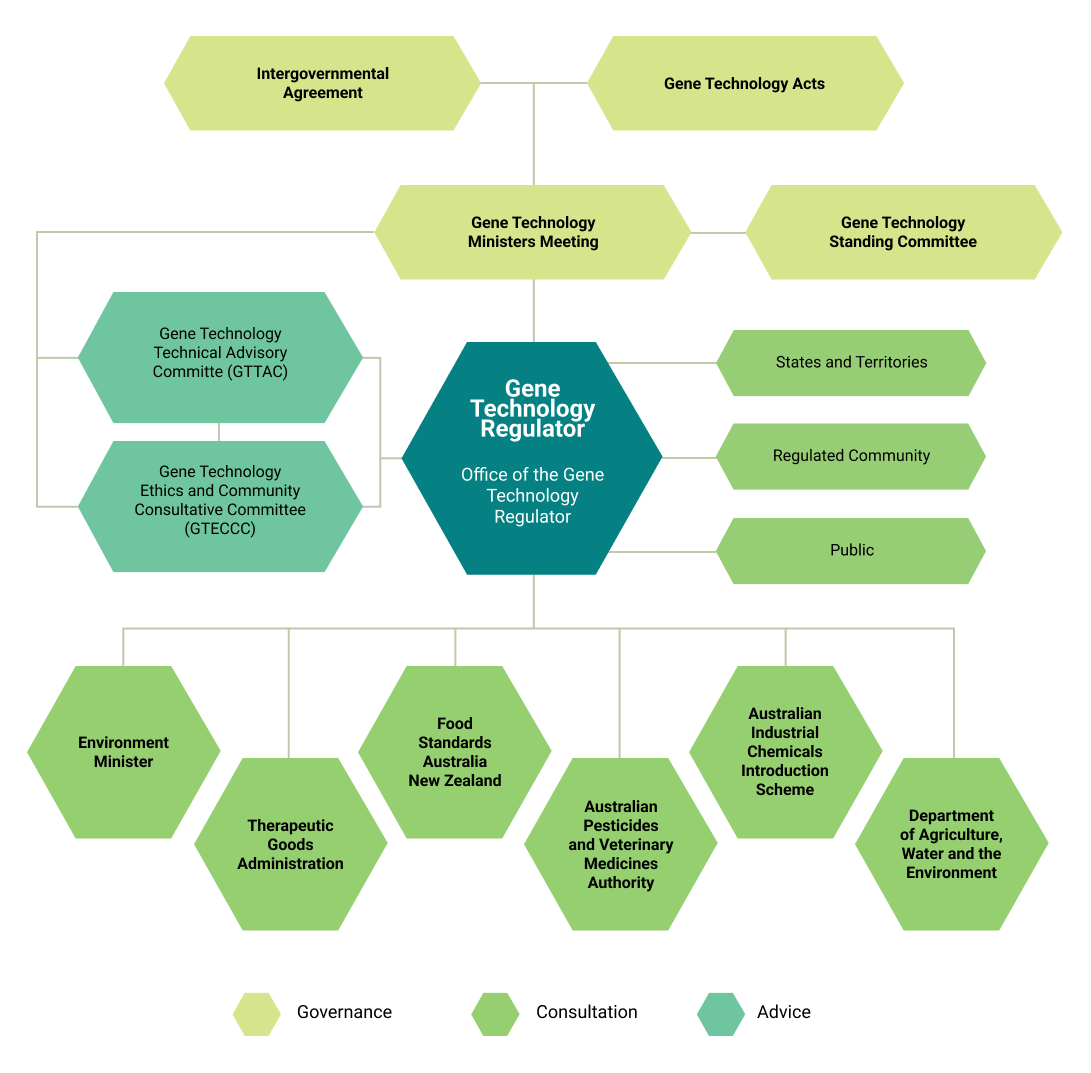
The Gene Technology Regulator (the Regulator) administers the legislation and makes decisions relating to all aspects of gene technology research and development in Australia, and relating to commercial releases of GMOs into the Australian environment.
The Regulator has a range of statutory functions which include:
- administration of the Commonwealth gene technology laws and corresponding state and territory laws
- monitoring, compliance and enforcement powers to regulate dealings involving GMOs.
The Regulator is governed by the Gene Technology Ministers Meeting (GTMM).The Minister for Agriculture and Food is the leading minister for gene technology in WA and represents the state at the GTMM. The GTMM is supported by the Gene Technology Standing Committee.
The department (DPIRD) leads the Western Australian Gene Technology Interdepartmental Committee. The committee has members from DPIRD, WA Department of Health, and the Department of Biodiversity, Conservation and Attractions. The committee provides a response to the Regulator as required during consultation on risk assessments and other proposals.
DPIRD is accredited to undertake GMO research and maintains an Institutional Biosafety Committee (IBC) as required under the national Scheme. The IBC is the local arm of the Office of the Gene Technology Regulator (OGTR) and has an oversight responsibility of DPIRD activites, reporting to the OGTR as required.
Gene technology is widely used to develop climate smart, pest and disease resilient crops, human medicines such as insulin to control blood sugars levels, and vaccines such as Gardasil to prevent human papillomavirus. It is also used to make vaccines for animals such as Hendra vaccine for horses to protect them from Hendra virus infections.
In Australia, no one may ‘deal’ with a GMO except under licence from the Regulator or as otherwise permitted under the Scheme. All dealings with a GMO that are not authorised for an intentional release into the environment must be carried out in a OGTR certified facility.
The National Gene Technology Scheme website provides more information about gene technology.
The content includes:
- how the Scheme operates
- how gene technology is regulated in Australia
- any relevant Ministerial announcements
- any updates to regulation and legislation including legislative reviews
- consultation opportunities and issues of interest in the future.
Contact us
-
Gene Technology Policy and Regulations

